In hopes of pushing the envelope of modern medicine, clinical trials are growing increasingly complex. With more interested parties, complexities take on an entirely different meaning.
Clinical trials typically involve five main stakeholders, and each group has its own views and motivations. Here is what people in those groups are saying about clinical trials from their own perspectives:
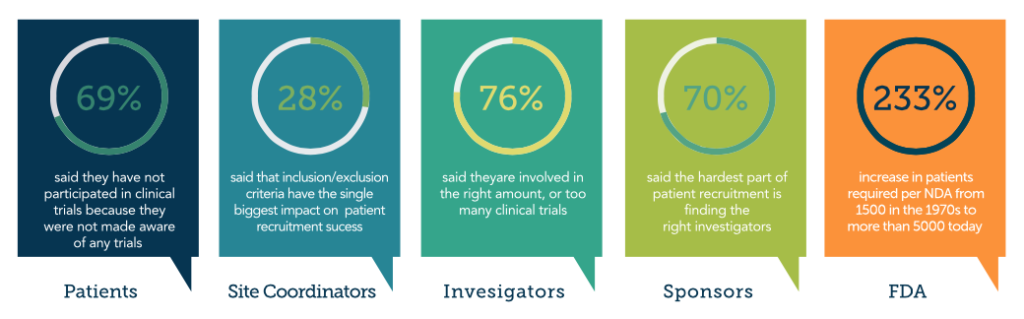
The 5 Faces of Clinical Trials
Patients
Most patients (69 percent) said they have not participated in a clinical trial because they were not made aware of any trials.
This tells us that our industry needs to do more to educate the public about clinical trials. Too many people are unaware that it’s an option for them. This is likely the reason whyrecruitment tends to be the number one reason studies do not finish on time.
Site Coordinators
Among site coordinators, 28 percent said that inclusion/exclusion criteria has the single biggest impact on patient recruitment success.
How can we put a program into place that can help sites expand their database and their reach?
Investigators
Most investigators (76 percent) said that they are involved in the right amount or too many clinical trials.
There are competing trials and we’re all vying for the same patient population. But we’re also vying for the site’s resources.
Sponsors
Most sponsors (70 percent) said the hardest part of patient recruitment is finding the rightinvestigators.
Big data and electronic health records are helping us get more savvy about identifying the right investigators. But more than 60 percent of investigators do only one or two clinical trials. With a constant churn of new investigators coming on board, additional training may be needed, and that adds to the timeline.
The FDA
The increase in patients required per drug application has soared 233 percent: from 1,500 in the 1970s to more than 5,000 today. This continues to put patient enrollment as the number one reason trials don’t complete on time.
Given the challenges across the five groups, “one size does not fit all” is a truism. Eachgroup has a unique role and set of challenges.