Accurate clinical trial translation services are key to the overall success of a study. Each participant needs to receive the same information in their native language to ensure consistent study messaging across all languages and accuracy to the protocol. I’ve compiled key translation best practices to consider when planning your global clinical trial and picking a translation vendor.
Where are clinical studies being done?
A good illustration showing the global nature of studies (and verifying the need for translations!) appears on clinicaltrials.gov. A healthy majority of studies listed there are non-U.S. only:

A table from the World Health Organization (WHO) shows us where trials are taking place:
Clinical Trials by Country or Region
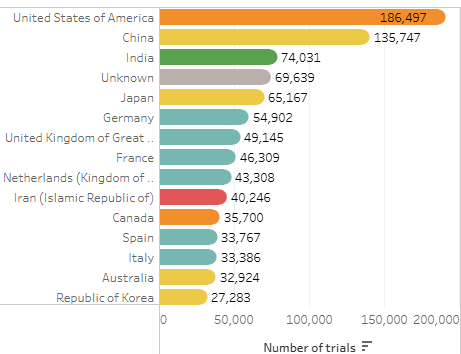
Clearly, to reach our participants, we need to translate clinical trial materials.
Translation in general vs. clinical research translation
Communicating with people in an understandable way is essential to clinical trial recruitment and retention, as well as participant safety and compliance throughout the study. It’s also a must according to some regulatory requirements.
While regulations vary from one country to another, clinical trial documents and communications must be in a language the clinical trial participant knows. Translating clinical trial materials into the local language allows participants to understand what the trial involves, promotes their choice to join the study, and enables their ability to follow trial instructions.
Because clinical trial materials are aligned with the protocol that has received regulatory approval, errors in translation can change the meaning of important content in study documents. This can cause protocol violations. Therefore, professional translators for clinical research need to have an excellent command of the language, and they also need to have a medical/scientific background to understand medical terminology and the protocol.
Translators must also understand the type of local clinical trial review process and the unique regulatory requirements for their region. And, finally, knowledge of local lingo is essential, so the participant materials are relevant and friendly to participants.
Begin with a strong foundation for a smoother translation process
Creating source clinical trial content using the principles of plain language, which is communicating in a way your audience can understand the first time they read or hear it, fosters source materials likely to invoke comprehension and engagement for all literacy and educational levels.
Also, thinking ahead and writing with translation in mind ensures the foundational text is unambiguous and easy to read. An improved source text makes it easier to translate. This saves time and money, avoids errors, and improves the readability of materials used for an international audience.
Essential qualities in your clinical trial translation team

To ensure you receive precise clinical trial translations, there are several qualities to look for when picking a translation vendor. First, make sure they have the expertise with all language pairs needed for your study. Next, the vendor should utilize qualified translators with experience in the life sciences. Translators should be selected based on their:
- Source and target language proficiency
- Educational and advanced training credentials
- Professional experience and certifications
Due diligence is important; assess their quality control processes to ensure they are robust. They should have:
- A trained and experienced project management team
- Effective standard operating procedures
- Continual quality improvement
- Strong document version control
- Proven outcomes
In addition, working with a translation vendor that adds value to the trial and collaborates with your in-country personnel (rather than just producing translation content as is) is a win-win for your trial and the participants. In-country clinical trial managers add valuable insight to the target-language content related to the specific study.
Machine translation
Over the years, machine translation has advanced, thanks to technological developments. The latest phase of neural machine translation is proving to be the most promising, providing the most fluid and accurate translation results to date. But this technology is still emerging.
As further data becomes available in more languages, machine learning will be able to produce more sophisticated outcomes. One challenge is to control the quality of the data used. Be sure to evaluate how a translation vendor utilizes this technology, control input quality, and check outcomes.
While machine translation is cost-effective, especially for handling large documents, it can stumble when handling cultural nuances and is typically unable to recommend appropriate local deviations from the source content. Best practices encourage human oversight of the outcomes at this time to ensure machine translations are accurate and patient-friendly.
There also remains a challenge of execution – we must not use machine translation as an incentive to accept substandard outputs to increase volume and keep costs low. (The money over quality dilemma exists everywhere, and the clinical trials industry is no exception.) However, as technology progresses, machine translation is likely to become an increasingly useful tool for clinical trial materials.
Imperial advantage
Imperial specializes in translation services for the global life-science industry, including the world’s largest pharmaceutical companies and CROs.
We provide:
- Global network of fully credentialed in-country linguists in over 100 languages
- Subject matter expertise
- Demonstrated history of success
- Quality process
- Certified quality management system to the ISO 9001:2015 standard by Intertek
- Demonstrated outcomes
- Strong document version control
- Notarized translation certificates
- Meets IRB/EC requirements
- Experienced, multilingual project management team
- Mitigates common pitfalls
- Coordinates with in-country trial managers to incorporate their insight
- Print-ready desktop publishing (DTP)/formatting
Imperial is a proven leader in the development, translation, print production, and global delivery of clinical trial materials. Visit our website for your clinical trial translation needs and to learn about our other exceptional clinical trial services.
Updated 29 SEP 2025

